Laboratory services
The ABO blood group system, along with the Rh blood group system (Rhesus), form what is commonly referred to as the blood group of a person. Antigens are proteins or polysaccharides on the surface of red blood cells.
When you request ABO grouping and blood group antibody screening on the request form to the Blood Bank, the samples are fully grouped into ABO and Rh blood groups and screened for blood group antibodies.
Full grouping consists of mixing a patient's red blood cells with anti-A, anti-B and anti-D antibodies. Testing for anti-A and/or anti-B antibodies in a patient's plasma, to match the red cell grouping, is also performed.
Blood group antibody screening consists of mixing red blood cells with known antigens and the plasma of the patient with 37°C and testing for agglutination. Such agglutination is an indication that the patient's plasma contains antibodies that bind to red blood cells.
Aside from the ABO and Rhesus systems, there are a number of other phenotypes with a lower relevance to blood component transfusion. However, they are naturally susceptible to the development of antibodies by patients given red cell concentrates or by women during pregnancy. The most important of these are Rhesus classes (C, E, c and e), Kell (K), Duffy (Fya, Fyb), and Kidd (Jka, Jkb).
In 1-3% of cases, the patients have antibodies to the phenotypic category (other than the ABO and Rhesus). For further analysis, see Blood Group antibodies. ABO classification
ABO blood group antigens are present on red blood cells and in fact most of the body's cells. Platelets express ABO blood groups, but to a lesser extent. The antigens in the ABO system are two, A and B.
The particularity of the ABO blood group system is that everyone develops antibodies to the A and/or B antigens they do not carry in their first year of life ("natural antibodies"). The antibodies to the ABO blood group system are anti-A, anti-B and anti-AB.
Croup (Phenotype) | Red blood cell antigen | Antibodies in plasma |
|---|---|---|
A | A | Anti-B |
B | B | Anti-A |
O | O | Anti-A, Anti-B |
AB | A and B | Neither |
Natural antibodies to the A and B antigens may be extremely active. When they bind to cells of wrongly transfused blood group, they can cause serious side effects and even death within a short time.
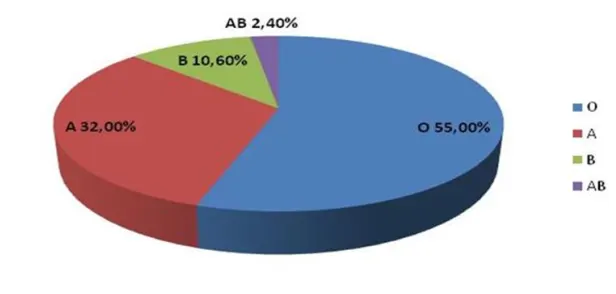
Percentage of ABO blood groups in Iceland:
Rhesus D grouping
Rhesus antigens are only present on red blood cells. Although the Rhesus blood group system consists of several antigens (e.g. D, C, E, c, e and Cw), only D is normally considered in the selection of red blood cell concentrate for transfusion. The formation of antibodies against Rh D is almost invariably a consequence of the immune response due to pregnancy or blood components and D is the most potent antigen known.
In Iceland, about 15% of individuals are RhD negative and about 85% are RhD positive.
Blood group antibody screening
is to evaluate if a patient has blood group antibodies. This is done by mixing the plasma of the patient with blood cells of known antigens. If the red blood cells clump, it is clear that the patient has developed antibodies to some of the antigens that are on their surface. The next step is to analyze the antibodies so that the patient can be given a red cell concentrate unit which does not carry the antigen.
Screening for antibodies is performed on all blood samples from patients that are sent to the Blood Bank and on samples from pregnant women with a maternal monitoring program.
A complete ABO grouping cannot be made for children under four months of age because they have not usually developed anti-A and/or anti-B antibodies.
Therefore, infant samples are only grouped using red blood cells, while plasma grouping on anti-A and anti-B is omitted.
Always perform a direct Coombs test (see "Direct Coombs Test" in the list below) on infant samples to check whether antibodies from the mother are bound to the baby's red blood cells.
The results of blood grouping and the direct Coombs test are found in Heilsugátt for those who have access to it or are notified by telephone to midwives/nurses/doctors at the relevant maternity institution. If the Coombs test is positive, the result is always notified by telephone.
The blood from the umbilical cord is blood grouped from the birth of all Rhesus D negative women in Iceland. If the blood from the umbilical cord is positive for Rhesus D, the mother is given anti-D immunoglobulin to prevent the development of anti-D antibodies.
The antibody that is administered binds Rhesus D positive red blood cells that may have entered the mother ́s bloodstream during placental rupture at birth. The mother ́s immune system cannot therefore get in contact with the anti-D antibody because it is bound to the anti-D immunoglobulin that was administered to her. The mother ́s immune system destroys the blood cells that are bound to the antibody.
Direct Coombs test
is always done immediately after the cord blood is grouped to check whether the mother's antibodies are bound to the baby's red blood cells.
The results of the blood grouping and the direct Coombs test are found in Heilsugátt for those who have access to it or are notified by telephone to midwives/nurses/doctors at the relevant maternity institution. If the Coombs test is positive, the result is always notified by telephone.
It is important to administer anti-D immunoglobulins to Rhesus D negative mothers of Rhesus D positive children within 72 hours of birth. If the results of the red blood cell grouping have not been received before that time, the woman should be given anti-D immunoglobulins to prevent the development of antibodies.
Compatibility testing includes verification of ABO and Rh D blood groups as well as blood group antibody screening. The blood group results are compared to known results of a patient on the blood bank computer system.
If no antibodies are detected and the blood group results are consistent with previous results, red blood cell concentrate may be reserved for the patient.
BAS and BKS are two types of compatibilty tests. The difference is that after a BAS test, a red blood cell concentrate can be reserved directly for the patient (called a computer cross-match test) but after a BKS test, a sample from the patient and the donor have to be cross-matched.
Prerequisites for BAS and a computer cross-match are that the computer system used is validated for the following:
Do not allow reservation of a wrong blood group for the patient
Patients who have been diagnosed with blood group antibodies are excluded from the computer cross-match
Computer cross-match cannot be performed on patients who have not been blood-grouped before
Patients with blood group disparities, positive DAT (direct Coombs test), and adverse reaction by blood components are excluded from the cross-match test unless they receive medical permission
Patients who do not meet the BAS criteria include:
Those who have been diagnosed with blood group antibodies
Those with a vague ABO and/or Rh-D blood group
BAS/BKS tests are valid for three days from the day of sample collection, therefore it is not possible to perform the BAS/BKS test on samples older than three days. BAS/BKS testing can be ordered without ordering red blood cell concentrate units. If a patient requires red blood cell concentrate at a later time, it can be ordered by phone or through Heilsugátt, for as long as BAS/BKS is valid.
It is possible to extend BAS testing in certain patients. In such cases, the BAS test is performed and set on hold for up to 10 days. As soon as blood products are reserved, the BAS test will be valid for a period of three days. However, the BAS test can never be valid for longer than the extension.
To extend BAS testing, you must fill in the appropriate box on the request to the Blood Bank and a doctor or his/her replacement must sign for it.
Criteria for extension of BAS test:
Patient must not have received blood products during the past 3 months
The patient must not be pregnant and has not been pregnant during the last 3 months.
Patient must not have received an organ in the past 3 months
Patient has never had a positive Coombs test
A Direct Coombs Test or DAT (Direct Antiglobulin Test) is a test for the presence of antibodies on the external surface of red blood cells in the body. This test is used e.g. when a patient's antibodies are suspected to cause breakdown of blood cells ("Immune Hemolytic Anemias").
The assay is based on the use of AHG (Anti Human Globulin) which can attach together the IgG antibodies on the surface of red blood cells so that the red blood cells clump together in a tube.

There are several reasons for the positive direct Coombs test, but here are a few examples:
Hemolytic Disease of the Newborn (HDN). Maternal IgG blood group antibodies can cross the placenta and bind to fetal blood cells carrying the antigen. The child's Coombs test will then be positive. The Rhesus anti-D is one of the most dangerous of these antibodies, but other blood group antibodies can also cause HDN depending on the severity of the antibody.
Hemolytic Transfusion Reaction (HTR). In transfusion, a patient may develop blood group antibodies. The blood group antibodies attatch to the donor cells and may cause an adverse reaction and a positive direct Coombs test.
Autoimmune and Drug-Induced Hemolytic Anemias (AIHA). Auto antibodies may bind to the patient's own blood cells, e.g. those with autoimmune diseases or haematological malignancies. Also, positive Coombs test can be caused by some medicines such as penicillin and methyldopa.
Screening for cold sensitive antibodies is a test to see if a patient has blood group antibodies that are only detectable in cold (at approximately +4°C). The plasma of the patient is mixed with red blood cells with known antigens and the mixture is left in the refrigerator for one hour. If agglutination occurs in the mixture, it is clear that the patient has formed a cold sensitive blood group antibody.
This type of screening is:
performed for open heart surgery where cooling is used during surgery. If a cold sensitive screening is positive, the blood group antibodies detected at room temperature (approximately +22°C) are also observed.
in some cases, used to support the diagnosis of blood group antibodies (see "Blood group antibody analysis" in the list below)
If antibodies are detected in the screening for blood group antibodies, the antibodies that are detected must be identified. Specific diagnostic methods based on the behaviour and nature of the antibodies are employed.
Immunoassays can be time consuming and in some cases require a significant amount of sample from the patient. Immunoassay consists of mixing the patient plasma with different red cells in relation to antigen. As part of the diagnostic process, a phenotypic grouping (see the list below on the page) will be performed on the patient and the antibody tested under different conditions such as different temperatures and the use of a catalyst.
Care should be taken not to give a patient, known to have a red cell antibody, RBC concentrate before the results of an assay are available.
This also applies to emergency blood!
Blood group antibodies can form during transfusion, during pregnancy or during childbirth if maternal-foetal blood mixing occurs. In pregnant women, antibodies in the plasma may cross the placenta and affect the foetus.
Maternal antibody quantification is of practical importance for antibodies capable of causing Hemolytic Disease of the Newborn or Hemolytic Transfusion Reaction (HDN/HDF). If the antibody is clinically relevant, assessment of the plasma antibody concentration should be performed at regular intervals to determine if the antibody concentration increases during pregnancy. This is done by a so-called titer measurement.
A titer measurement does not reveal the actual amount of antibody, but indicates whether the antibody concentration is increasing since the concentration is always compared to an earlier mother sample.
Specialists at Landspítali University Hospital will always receive a report of clinically important antibodies.
When grouped into blood groups other than ABO and Rhesus D, it is referred to phenotyping.
Patients with an autologous disease that require repeated blood transfusions are often grouped into 13 phenotypic categories. This is to help detect future blood group antibodies. Since RBCs transfused from a RBC concentrate survive for 3-4 months in the recipient's circulation, it is not possible to phenotypically classify the recipient for three months after transfusion.
The main phenotype classes are:
Rhesus antibodies other than D
The main clinically relevant antibodies, apart from anti-D, belonging to the Rhesus system are C, E, c, e and Cw.
Clinically relevant antibodies against C, E, c and e classes are listed, although the antigens are less antigenic than Rhesus D. They may all cause severe adverse reactions to transfusion and Hemolytic Disease of the Newborn.
Patients with blood group anti-C, anti-E, anti-c or anti-e antibodies will always receive a red blood cell concentrate which does not carry the antigen in question.
Anti-Cw may be clinically relevant. This is a common antibody and can be natural, i.e. in individuals without known exposure to the antigen. A cross-match is used when selecting red cell concentrate in patients with anti-Cw (i.e. it is not necessary to find a known Cw negative unit).
Anti-K (Kell) and anti-k (Cellano)
K is an antigen that is highly antibody-inducing. It is thought to be closest to the D antigen in this respect. About 90% of individuals have a K negative status.
The anti-K antibody is clinically important and may cause adverse reactions after transfusion, severe Hemolytic Disease of the Newborn, and embryonic death. It seems that this antibody can cause normal formation of red blood cells in a foetus. Therefore, women in pregnancy who have anti-K and K positive father of child are followed very closely.
Women of childbearing age who do not carry K antigen are given K negative erythrocyte concentrates to prevent formation of K antibodies.
Anti-Fyaand anti-Fyb(Duffy antidote)
Two antigens belong to the Duffy blood group system, called Fya and Fyb (Duffy A and Duffy B).
Antibodies to these antigens are clinically important and may cause sudden side effects after transfusion and Hemolytic Disease of the Newborn in children of antibody-positive mothers. About 32% of individuals are Fya negative and about 20% Fyb negative.
Almost no Caucasian individuals are negative for both Fya and Fyb blood group. However, this phenotype is common in black individuals and it probably evolved this way because the Duffy antigens act as receptors for the malaria parasite. Therefore, those who do not have the Duffy antigens will have less malaria.
Anti-Jkaand anti-JkbAntibodies (Kidded antibodies)
The antigens belonging to the Kidd blood group system are known as Jka and Jkb.
Anti-Jka and anti-Jkb antibodies are extremely dangerous. They can form through blood transfusion, blood mixing during pregnancy or childbirth. The antibody is quickly removed from the circulation, making it difficult to detect. However, if a patient develops blood with the antigen at a later time, the antibody level may increase again and cause a delayed reaction which can be very serious.
These antibodies rarely cause Hemolytic Disease of the Newborn.
Anti-M, anti-N, anti-S, anti-s
Antibodies to the M and N antigens are generally not clinically relevant. They are generally IgM type and cold sensitive. IgM antibodies do not cross the placental barrier.
If these antibodies are detected at 37°C and are IgG-type they can be clinically important. Patients with such antibodies are given a red blood cell concentrate which does not carry the antigen in question.
Anti-S and anti-s antibodies are less common and are more likely to be clinically relevant. These antibodies have been implicated in serious adverse reactions following transfusion. Some cases of neonatal jaundice caused by anti-S and anti-s antibodies have been reported.
Rhesus D negative women are offered Rhesus D screening for the fetus at 24-26 weeks. If a foetus is diagnosed with Rhesus D positive, mother is offered anti-D antibodies (immunoglobulins). This antibody binds to the foetal blood cells in the mother's bloodstream and destroys them. Thus, the administration of antibodies prevents the mother from initiating antibodies to foetal red blood cells.
Method:
EDTA blood sample is collected from the mother at 24-26 weeks of pregnancy. The sample is spun to eliminate the blood cells. Short DNA fragments from the mother and foetus are found in the plasma from broken cells. Generally, 10-30% of the DNA fragments are derived from the foetus (placental degradation products). To screen for Rhesus D, DNA is isolated from the plasma and a specific qPCR amplification of the Rhesus D gene is performed. Regression of the Rhesus D gene is the most common cause of Rhesus D negative genotype among Europeans. If none of Rhesus D increases from the sample, then both mother and foetus have Rhesus D negative. However, if the gene is amplified, it indicates that the fetus does not have the deletion and thus has a Rhesus D positive. Rhesus D negative women, who have Rhesus D negative foetuses, do not need to have an anti-D antibody injection.
Generally, Rhesus D screening are performed weekly. The answers appear in Heilsugátt 1-2 weeks after samples are sent for testing.

Tissue groups are molecules found on the exterior of most cells of the body. The concentration of these molecules is highest on white blood cells. Human leukocyte antigens have been termed leukocytes because they were first found on white blood cells. The acronym HLA stands for these molecules and is widely used.
The HLA molecules play an important role in the body's immune response. If an infection occurs in the body, a peptide that originates from the pathogen is broken down in the cell and bound to HLA molecules. The cells of the immune system 'feel' for the HLA molecules and if the cells of the immune system find an unknown peptide originating from, for example, a bacteria or a virus, the immune system will be activated.
To be able to detect the greatest number of unknown peptides, many types of HLA molecules are needed. Therefore, there are several genes that determine these molecules, each gene has a high degree of variability and this leads to the existence of multiple versions of these molecules.
Two protein complexes are the major targets for tissue group determination. Tissue groups are divided into class I and class II according to the type of these protein complexes.
Class I: Only one of the protein complexes is determined by tissue class molecules and has high variability. The other protein complex is called β2-microglobulin, which originates from a different gene region. There is no variability in the β2-microglobulin protein complex.
Class II: Both protein complexes are determined by tissue class molecules, but only one, DRβ1, has significant variability.

Genes that store information for tissue groups are on chromosome 6. The region containing these genes is located in the most gene-rich region of the human population and contains many genes related to the immune system.
The genes are close to each other and therefore often inherit together as a single unit or haplotype. Family research can analyze how these haplotypes are inherited between parents and children.
Each child inherits one haplotype from each parent. Since each parent has two haplotypes (one on each chromosome), there is a 50% probability that two children of the same parents will have the same haplotype from the same parent.
Since the possibilities of a combination of the parent haplotypes are four, there is a 25% probability that two children of the same parents have both haplotypes in common and also a 25% probability that they have no haplotypes in common, see figure.
The most relevant tissue groups are: HLA-A, HLA-B and HLA-DR. The genes that register for these proteins have a high degree of variation, which is necessary for these molecules to recognize the largest number of unknown peptides from pathogens.
The wide variation implies that there are multiple versions of these proteins, so there is little chance that two unrelated individuals will have the same type of tissue classes.
Great variability also implies that it can be difficult to distinguish between different tissue groups because the number of known molecules is high and the techniques used for classification must be able to distinguish between them.
When and why tissue grouping
Tissue grouping are performed in conjunction with renal and stem cell transplantation. Live donor kidney transplantation was initiated in Iceland in late 2003 and the blood bank's tissue grouping department has carried out important preliminary studies for these procedures. Stem cell transplantation is not performed but the tissue grouping department performs tissue grouping on the patient and his or her family and the results of tissue grouping determine whether a family member undergoes further research.
Tissue grouping are also performed as part of the diagnosis of certain diseases that are strongly associated with certain types of tissue groups. Best known in this context is the spondyloarthritis which is strongly associated with the tissue class B27.
The Icelandic Blood Bank has been in cooperation with the Norwegian register of bone marrow donors since 2004. It is a list of tissue grouped blood donors that have declared themselves ready to donate stem cells to unrelated parties. These donors are grouped and information, along with information on blood type, age and gender, are housed in the Norwegian Bone marrow Donor Register. If a patient requires a stem cell transplant is found to have no relatives with the same tissue group, a search for a tissue grouped stem cell donor is conducted.
The department tissue grouping has participated in research on diseases where a link to certain tissue groups is suspected.
What does the Blood Bank Tissue Grouping Department do?
Tissue grouping:
Tissue grouping at the Blood Bank is based on grouping for A, B and DR sites. Tissue grouping is made using DNA techniques. DNA is isolated and then certain areas of the genetic material are amplified and analysed with specialized technology and software.
Genes that determine an individual's tissue group, have a high degree of variability. This means that there are many versions of the molecules that determine tissue class. The technique used in the analysis must be able to distinguish these different molecules with some certainty. This is therefore a very specialized and complex technology, and the blood bank has technology which is one of the best in the world.
HLA cross-match:
HLA cross-match are performed between donors and recipients to evaluate whether the recipient has antibodies to the donor tissue classes. In the case of antibodies, the recipient's immune system immediately rejects the organ. HLA cross-matches are performed by isolating donor lymphocytes and mixing the patient sera. If recipient specific antibodies are present, they bind to cells from the donor. Complement is added and after antibody and cell binding, cytodependent complement killing occurs and microscopically colour differences between dead and living cells can be detected.

HLA antibody screening:
HLA antibody screening is used to examine if antibodies are detected against the molecules determining tissue group. Because many tissue group molecules exist, it is often difficult to identify the antibodies. Since 2006 the Icelandic Blood Bank has a novel technology that can detect these antibodies with great sensitivity.
Patients awaiting transplantation are screened for HLA antibodies because it is not desirable that potential donors have the same tissue group molecules on the surface of their cells as the recipient has antibodies against them.
The surface of platelets contains tissue group molecules. The immune system of patients who have antibodies to these molecules destroys platelets with antigens they have antibodies to. Therefore, it may be necessary to identify the antibody and select a platelet donor on the basis of these results.
Research Results
Appear in Heilsugátt and Interinfo (a portal into the Blood Banking System).
The results of blood grouping and screening for blood antibodies appear within three days of the sample being delivered to the Blood Bank.
If the individual has or is diagnosed with a blood antibody, the response will appear within a week.
More info on (pdf)
